Novel Study: The Little Prince
The Little Prince meets a conceited man, a drunkard, a
businessman, a lamplighter and a geographer.
He comes to know the absurdity or ridiculousness of
adults. He believes all work must serve a purpose, have meaning and be truly useful to others. We are not be self-centred but rather
caring and loving.
What is your opinion of each of these characters that he
meets?
Why would the Little Prince compare grown-ups to Baobabs?
What’s he trying to say?
What impression do you have of the snake?
Why do you think the inconsequential flower the Prince
meets in the desert tells him that people don’t have roots and tend to drift
about?
Remember to write in 1st person point of view when offering a personal opinion i.e. I feel... I think... I believe... I'm surprised by... I wonder... I can't understand why... I learned the importance of... plus 1 supporting reason
Science Lab 5: Solutes vs Solvents (text pg.41)
A solvent, the large part of a solution, in which the solute dissolves.
i.e. sugar (solute) dissolves in hot coffee (solvent) because particles of each are strongly attracted to one another.
Purpose: to compare how sugar (solute) dissolves in 3 different solvents - water, rubbing alcohol and oil.
Hypothesis:
1. If I dissolve sugar (solute) in water (solvent), then I think the sugar particles with be very attracted to/somewhat attracted to/unattracted to the water particles and will dissolve quickly/somewhat quickly/not at all.
2. If I dissolve sugar (solute) in rubbing alcohol (solvent)... same as above.
3. If I dissolve sugar (solute) in oil (solvent)... same as above.
Observations:
Experiment Rate of Dissolution
Sugar in water
Sugar in oil
Sugar in rubbing alcohol
Analysis:
Questions B & C pg.41
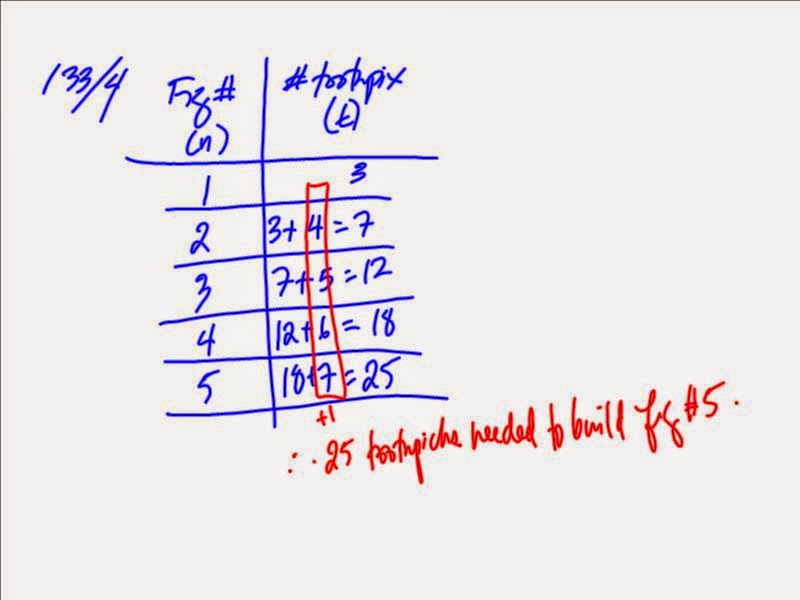
Patterns/Relationships/Table of Values/Sequences
Remember to write in 1st person point of view when offering a personal opinion i.e. I feel... I think... I believe... I'm surprised by... I wonder... I can't understand why... I learned the importance of... plus 1 supporting reason
Science Lab 5: Solutes vs Solvents (text pg.41)
A solvent, the large part of a solution, in which the solute dissolves.
i.e. sugar (solute) dissolves in hot coffee (solvent) because particles of each are strongly attracted to one another.
Purpose: to compare how sugar (solute) dissolves in 3 different solvents - water, rubbing alcohol and oil.
Hypothesis:
1. If I dissolve sugar (solute) in water (solvent), then I think the sugar particles with be very attracted to/somewhat attracted to/unattracted to the water particles and will dissolve quickly/somewhat quickly/not at all.
2. If I dissolve sugar (solute) in rubbing alcohol (solvent)... same as above.
3. If I dissolve sugar (solute) in oil (solvent)... same as above.
Observations:
Experiment Rate of Dissolution
Sugar in water
Sugar in oil
Sugar in rubbing alcohol
Analysis:
Questions B & C pg.41
Patterns/Relationships/Table of Values/Sequences